Product Introduction Management
The gold standard for new product evaluation
Evidence-based device selection, integrated workflow for device request management and collaboration tools for value analysis committees.
Book a Demo
Product Introduction Management


Web-based Product Introduction Management enables a modernized product review that engages physicians, standardizes processes and ensures that product approvals are limited to only those products that have demonstrated improved clinical value.
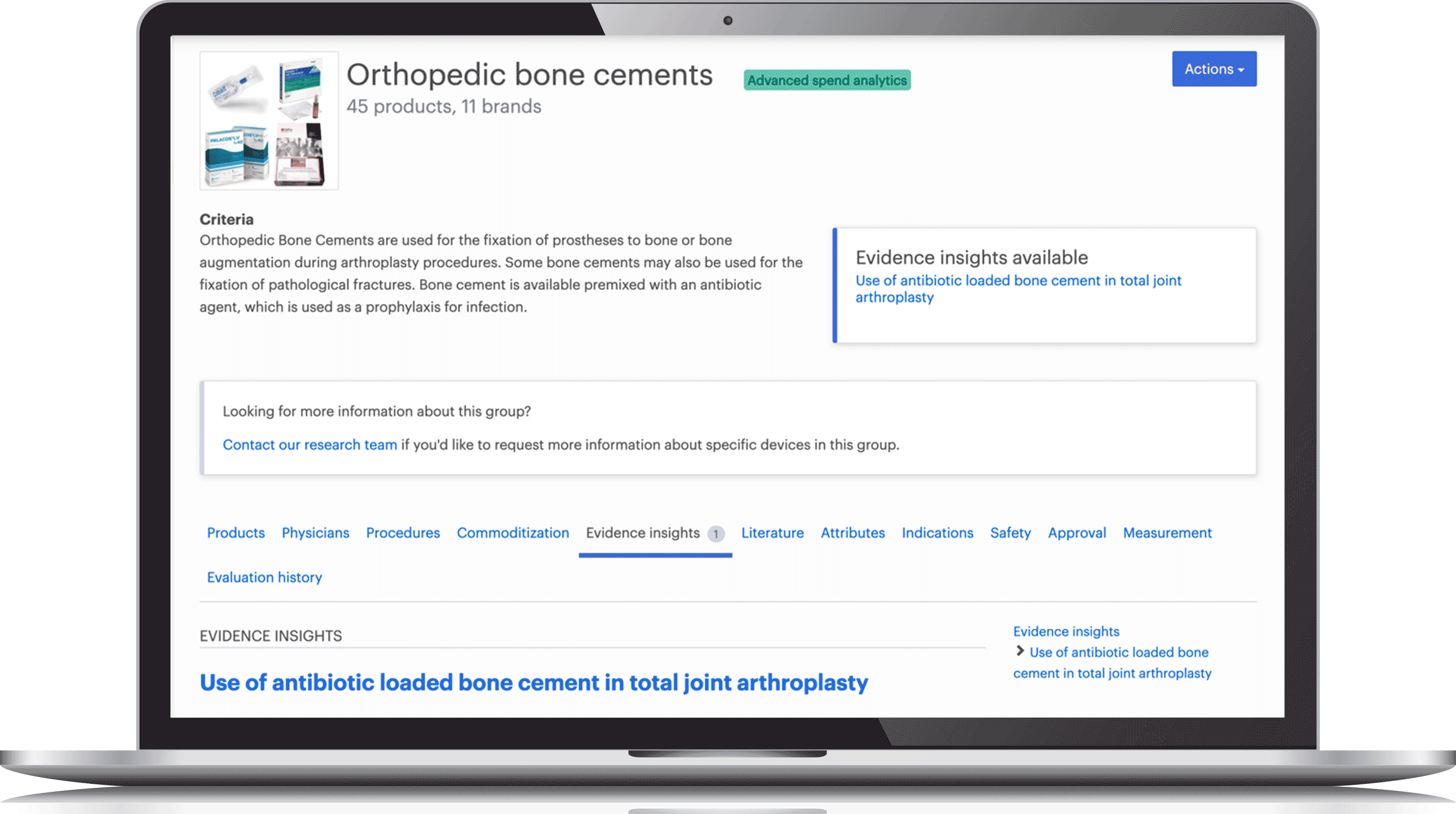
Access comprehensive evidence reviews that address efficacy, safety and appropriate utilization, along with the underlying research articles, indexed by level of evidence and manufacturer sponsorship.
Cross-reference over 36,000 devices based on functional similarity. Compare products based on their specs, costs, level of differentiation, safety profiles and clinical outcomes.
Analyze medical devices from both a cost and clinical perspective to find common ground and build trust with physicians.

Automate your process and ensure that your committees are organized and armed with the key information to make evidence-based decisions.
Deliver relevant research to physicians via email, collect feedback through online voting and proactively keep them informed of the status of their requests.
Organize the right mix of committee members, experts and evidence to assess the organizational impact of your decisions.
“By providing objective, up-to-date,peer-reviewed literature, Product Introduction Management enables meaningful conversations and elevates the discussion from being about just devices to being about delivering the best patient care.”
Susan G. Miller, RN, MN, CMRP, CVAHP, Senior Director, Value Analysis, Jefferson Health
By submitting my information here, I consent to GHX entities and their service providers processing my information to respond to my request, including transferring my data to other countries, as described in the GHX Privacy Policy.